

|
Field/Button |
Status |
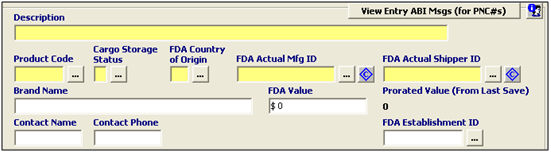
Description |
|
View Entry ABI Messages (PNC#) |
Button |
Click to view the entry ABI messages. This is used to locate your prior notice confirmation numbers (if available) for this entry. |
|
Description |
Mandatory |
Enter the FDA commercial description which describes the product for FDA purposes. |
|
Product Code |
Mandatory |
Enter the FDA product code for the product, which is the code that identifies the FDA product. Click the ellipsis to use the FDA Product Code Builder. |
|
Cargo Storage Status |
Mandatory |
Enter the cargo storage status, or choose from the pick-list by clicking the ellipsis. This code describes the status of the product when it is shipped. |
|
FDA Country of Origin |
Mandatory |
Enter the FDA country of origin, or choose from the pick-list by clicking the ellipsis. Please note that the FDA C/O may differ from the C/O reported to US Customs. |
|
FDA Actual Manufacturer ID |
Mandatory |
Enter the MID for the actual manufacturer, or choose from the transaction parties list by clicking the ellipsis. |
|
FDA Actual Shipper ID |
Mandatory |
Enter the MID for the actual shipper, or choose from the transaction parties list by clicking the ellipsis. |
|
Brand Name |
Conditional |
Enter the brand name of the product, which is required for radiation emitting devices. |
|
FDA Value |
Conditional |
Enter the total value of the line for FDA purposes. |
|
Prorated Value |
Displayed |
This area will display the prorated value for this line (from the last save). |
|
Contact Name |
Conditional |
Enter the name of the party that is completing the prior notice. This is the party that the FDA will contact for information/requests/questions. |
|
Contact Phone |
Conditional |
Enter the phone number of the party that is completing the prior notice. This is the number where the contact party can be reached for more information. |
|
FDA Establishment ID |
Conditional |
Enter the FDA Establishment ID, if required. This identifies the final destination of the product. Required if the CBP consignee is foreign based. |
|
Field/Button |
Status |
Description |
|
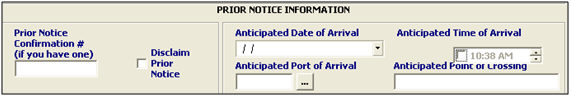
Prior Notice Confirmation # |
Optional |
Enter the Prior Notice Confirmation number if one has already been obtained. This number is issued by the FDA to confirm that they have received the prior notice data. |
|
Disclaim Prior Notice |
Checkbox |
Check this box to disclaim the prior notice portion of this FDA transmission. This indicates that the prior notice reporting is not required for the product. |
|
Anticipated Date of Arrival |
Mandatory |
Enter the date of arrival at the port of entry for this transaction, or click the drop-down to choose from the calendar. This will auto-create the affirmation of compliance for this data. |
|
Anticipated Port of Arrival |
Mandatory |
Enter the anticipated port of arrival for this shipment, or choose from the pick-list by clicking the ellipsis. This will auto-create the affirmation of compliance for this data. |
|
Anticipated Time of Arrival |
Mandatory |
Enter the anticipated time of arrival for this shipment at the port of arrival. This will auto-create the affirmation of compliance for this data. |
|
Anticipated Point of Crossing |
Mandatory |
Enter the anticipated point of crossing, if it differs from the default that fills in from the selection of the port of arrival. This will auto-create the affirmation of compliance for this data. |

|
Field/Button |
Status |
Description |
|
Submitter Information |
Mandatory |
Enter the firm and contact name and address for the submitter of this prior notice information. The system will automatically create affirmations of compliance from this entered information. |
|
Transaction Parties |
Buttons |
Click the Transaction Parties button to auto-fill this information from a transaction party, or click the “bring in from profile” button to bring in the information from the main profile screen. |
|
Profiles |
Button |
Click to bring the information in from a client profile. This will open the client search window, where you can locate the desired profile. |
|
Owner Firm Type |
Conditional |
Enter the firm type for the owner of this cargo, or choose from the pick-list by clicking the ellipsis. |
|
Country of Shipping |
Conditional |
Enter the ISO code for the country of shipping, or choose from the pick-list by clicking the ellipsis. |
|
Mfg/Processor FDA Registration # |
Conditional |
Enter the FDA Registration number for the manufacturer. This is the 11 digit number that indicates that the manufacturer has registered with the FDA. This field is not required if you are selecting an exemption code. |
|
Mfg/Proc. Firm Type |
Mandatory |
Enter the firm type for the manufacturer/processor, or choose from the pick-list by clicking the ellipsis. |
|
Mfg/Proc. Registration Exemption Code |
Optional |
Enter the manufacturer/processor exemption code, if this manufacturer/processor is exempt from FDA registration requirements. If you are entering an exemption code, you can leave the FDA registration number blank. |
|
Shipper FDA Registration # |
Mandatory |
Enter the FDA Registration number for the shipper. This is the 11 digit number that indicates that the shipper has registered with the FDA. |
|
SCAC & Master Bill#/AWB# |
Mandatory |
Enter the SCAC/IATA and the master bill, pro bill, or air waybill for this shipment. |
|
Field/Button |
Status |
Description |
|
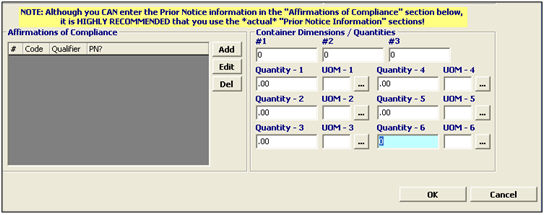
Add |
Button |
Click to add a new affirmation of compliance. This will open a new item in the appropriate screen. |
|
Edit |
Button |
Click to edit the selected affirmation of compliance. This will open the selected item in the appropriate screen. |
|
Del |
Button |
Click to delete the selected affirmation of compliance. |
|
Container/Dimensions (#1, #2, #3) |
Conditional |
Enter the container measurements (acidified and low acid canned foods): - Container is rectangular - dimensions are width(1), height(2), length(3). - Container is cylindrical - dimensions are diameter(1), height(2). |
|
Quantities |
Conditional |
Enter the quantities associated with the product. This identifies the packaging of the product. You must report each container/package quantity, decreasing from the largest container to the smallest (base unit/quantity). |
|
UOM |
Conditional |
Enter the units of measure associated with the quantities, or choose the UOM from the pick-list by clicking the ellipsis. The smallest must be a base unit of measure. FDA UOM codes may differ from US Customs UOM, so please verify the codes that are being used. |
|
OK/Cancel |
Buttons |
OK will save the FDA Information. Cancel will cancel and go back to the OGA screen without saving. |